While often overlooked, the tag is the major means through which a manufacturer conveys important information to you regarding the product, such as the details necessary for its safe and effective use.
While this information may differ slightly across state lines, even for the same product, certain important information is contained on almost all tags in the U.S. The following is an overview of the major sections on a tag, and more importantly, what those sections have to say.
Name
The product name and its branded name (when applicable) are generally the first information at the top of the feed tag and often provides an indication of the feed’s purpose. As product names become more specific, the amount of additional information provided on the tag generally increases in order to support that degree of specificity. If the feed contains a drug, making it “medicated,” the tag should also contain a statement describing the product as either a Type B or Type C medicated feed. As a reminder, Type B medicated feeds cannot be fed directly to animals – they must be further blended to produce a Type C medicated feed that contains the appropriate (approved) level of drug before being fed. If the medicated feed contains a veterinary feed directive (VFD) drug, a statement that serves as a reminder of the necessity for veterinary involvement will also appear beneath the product name.
Purpose
The purpose statement is a brief description of the intended purpose of the feed or ingredient. More specifically, this statement may provide species- and animal class-specific information pertaining to the animals the feed should be fed to or how it should be used to further manufacture feed. This information may be complex or quite vague, depending upon the breadth of intended uses for the product. If the product name provides this information, the purpose statement may be absent from the feed tag.
Indications for use and active drug ingredients
Medicated feeds are required to contain an “indications for use” statement, along with a listing of the drugs used to medicate the feed. This information generally appears between the purpose statement and guaranteed analysis section for medicated feeds but is absent from nonmedicated feeds. The indications for use statement contains the specific approved use of the drugs, as it applies to that particular product.
For example, a medicated feed that lists amprolium as the active drug ingredient may list the indications for use in calves as an aid in the treatment of coccidiosis caused by Eimeria bovis and E. zuernii, which is one of the two approved uses of that drug to medicate feed for cattle. Drug concentrations are generally listed to the right of the active drug ingredients and expressed in either grams per ton (g/ton) or grams per pound (g/lb).
Guaranteed analysis
The guaranteed analysis section conveys information pertaining to the maximum and/or minimum amount of a respective nutrient the product is guaranteed to contain, referenced in nutritionally relevant units. The requirements for nutrient guarantees differ across animal species and production classes, as well as the intended use of the feed, or other information provided on the tag.
For example, guarantees generally differ for complete feeds when compared to mineral supplements. Because the intended use of a mineral supplement is to serve as a supplemental source of minerals and vitamins, mineral tags generally don’t contain guarantees for other nutrients.
But the tag for a mineral supplement that is also intended to serve as a source of supplemental crude protein – for example – generally guarantees a minimum level of crude protein. Depending upon the nutrient, and some of those other factors, guarantees may only be minimum levels, maximum levels or both. If a source of nonprotein nitrogen such as urea has been added, the equivalent amount of crude protein from non-protein nitrogen is stated below the crude protein guarantee.
For beef cattle, macronutrients (crude protein, crude fat, etc.) are generally expressed as a percentage by weight, which is also commonly referred to by nutritionists as being expressed on an as-fed or as-is basis. Micronutrients are a little different.
Trace minerals are generally guaranteed in parts per million (ppm; 1 ppm = 1 milligram per kilogram = 0.0001%) when the guaranteed level is less than 1% (10,000 ppm). Fat-soluble vitamins (A, D, E and K) are guaranteed in international units (IU), and depending upon level, may be expressed in kilo-international units (KIU; 1,000 IU = 1 KIU). If guaranteed, vitamin B12 is generally listed in micrograms per pound (mcg per pound), while other water-soluble vitamins are listed in milligrams per pound (milligram per pound).
Feeds and ingredients also contain nutrients that are not guaranteed. More guarantees generally equate to less manufacturing and pricing flexibility. Do not expect a tag to paint the full picture of a product’s nutrient profile. Energy values are a prime example of incredibly useful numbers that are rarely guaranteed on the tag. And unfortunately, there are rarely enough other nutrient guarantees to reliably calculate expected energy values.
If you’re looking for nutrient information beyond what is provided by the tag, your best bet is to request an expected nutrient profile from the product representative. Just keep in mind, they may not always be willing to provide that information. Additionally, medicated feeds may also guarantee certain physical properties or aspects of the product, depending upon the requirements stated in the drug’s approval.
List of ingredients
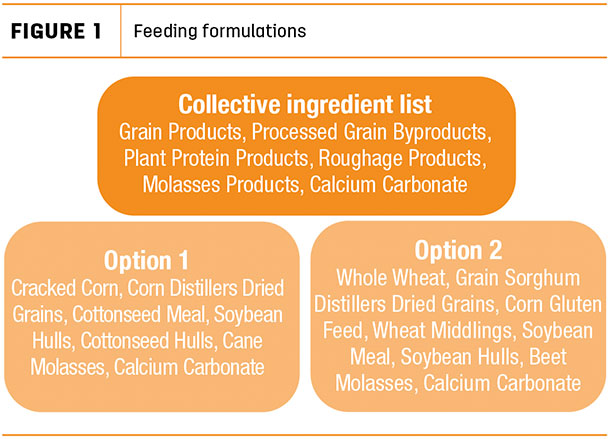
This is the portion of the tag that conveys the ingredients included in the product, which is essentially the formulation without the numbers. Ingredients are always listed in descending order based upon their inclusion rate. Manufacturers have the freedom to list the actual defined ingredient name or to list certain ingredients under more ambiguous collective terms in order to preserve the product’s proprietary formulation. To illustrate this, the collective ingredient list provided in Figure 1 is an example of an ingredient statement that describes the formulations used to manufacture options 1 and 2.
Directions for use
This section provides directions to ensure the safe and effective use of the product. Unfortunately, this portion is often overlooked. Following the directions listed on the tag allows the product to be used in a way that achieves the product’s purpose. A prime example of this is the very common question of how to regulate consumption of free-choice, loose mineral supplements when cattle are over- or underconsuming the product, which is often explained in the directions for use section – to move the mineral feeder.
This section also contains further mixing directions if the product is intended to be mixed with other feeds or ingredients prior to being fed or if other feeds or ingredients should be provided to cattle fed the product. This is particularly important for medicated feeds intended for further mixing prior to feeding. For some products, this section may direct you to another portion of the tag or package to find additional information on how the product should be used.
Warning and caution statements
The warning statement provides information about the product that pertains to human safety concerns. This may include withdrawal times for medicated feeds, as well as any concerns or precautions related to mixing, handling or feeding the product. The caution statement, on the other hand, provides information pertaining to animal health or safety.
This may include certain animal species or production classes that should not be fed the product or certain mixing or handling directions related to animal health or safety concerns. A prime example of this is the statement to avoid providing horses or other equines with access to feed containing an ionophore, as it may be fatal.
Name and address of manufacturer
This section provides the name and contact information for the party responsible for the product. Feeds or ingredients manufactured by another party – or “toll-milled” – for the party that marketed the product are generally signified by a phrase such as “Manufactured for …” or “Distributed by …”.
Quantity statement
The last section, which is generally found at the bottom of the tag, is the quantity statement, which describes the amount contained in the package or bulk load. This amount is expressed in both standard (pounds or gallons) and metric (kilograms or liters) units.
Reviewing a feed tag can provide you with an enormous amount of information on a product. This information can serve as a valuable tool when deciding what products will be the best fit for your operation. If you haven’t recently done so, take some time to review your feed tags, as you might be surprised to find the amount of information already at your fingertips. And if any of it is unclear or doesn’t make sense, don’t hesitate to ask for clarification. ![]()
PHOTO: Be mindful of label instructions that tell you how to deliver the product, either by itself or with other ingredients. Staff photo.

-
Jason Smith
- Extension Beef Cattle Specialist
- Texas A&M University, AgriLife Extension
- Email Jason Smith









